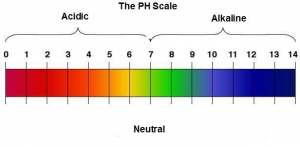
There is much confusion around pH, alkaline and alkalising.
ph is the abbreviation for “pondus Hydrogenium“. Normally it is referred to as “Potential of Hydrogen”. This literally means the weight of hydrogen as is an indication of the acid levels in a substance.
The pH of water for example may be 9.5 and the alkalinity may be Low, Moderate or High.
It’s not high pH alkaline water we need, it’s water with high levels of ALKALINITY. -Dr Marc Sircus.
pH is not as we may think when we are working to alkalize the body (“think” lemon juice, apple cider vinegar and fresh green juices – all of which have an acid pH and are still great for alkalizing!). Addressing excessive metabolic acids and building alkalizing reserves in our body fluids and tissues relates to alkalinity (alkaline mineral compounds). We can drink a sparkling mineral water with a pH of 6.5 you can alkalise the body.
Water expert Robert Slovak wrote, “Baking soda is still beloved! It delivers the goods – real alkalinity – while most popular alkaline pH drops from well-known “health experts” are a hoax at $35-$40 for pennies worth of caustic chemical in 2oz of water. Just note that for some people baking soda (sodium bicarbonate) may supply too much sodium so they can substitute potassium bicarbonate. High pH ionized water, without accompanying alkalinity (bicarbonates, carbonates and hydroxides), does little but waste money.” High pH ionized water, without accompanying alkalinity (bicarbonates, carbonates and hydroxides), does little but waste money.” continued Slovak.
Robert Slovak goes to suggest “The truth is, when atmospheric CO2 reaches equilibrium in distilled or RO (reverse Osmosis) water (forming weak carbonic acid) the pH goes slightly lower (say pH 6.5) but there is virtually no acid-buffering capacity! As soon as the distilled or RO water hits your saliva or stomach-acid, its pH is rapidly readjusted and it takes on a different pH and buffering capacity. To say that distilled water and RO water are highly acidic is simply wrong – a marketing gimmick to favor alkaline water! The weak carbonic acid that forms simply cannot acidify the body. Furthermore, just a little baking soda dust on the end of your finger can completely neutralize a glass of distilled or RO water.
Dr Marc Sircus suggests “Alkalinity is the water’s capacity to resist changes in pH that would make the water more acidic. This capacity is commonly known as “buffering capacity.” For example, if you add the same weak acid solution to two vials of water – both with a pH of 7, but one with no buffering power (e.g. zero alkalinity) and the other with buffering power (e.g. an alkalinity of 450 mg/l) – the pH of the zero alkalinity water will immediately drop while the pH of the buffered water will barely change at all.”
The alkalinity of natural water is determined by the composition of soil and bedrock which is moves through. especially when it contains carbonate, bicarbonate, and hydroxide compounds. borates, silicates, and phosphates. Limestone is good example of a source for alkalising water. Surface water (e.g., streams, ponds, lakes) and underground zones rich in granites, some conglomerates and sandstones may have low alkalinity and therefore poor buffering capacity.
A good source of buffering minerals is the key to alkaline water. Water with low buffers may read with a high pH when run through an ionising filter but has a low alkalising ability.
Alkalinity is a measure of the capacity of water to neutralize acids. It measures the presence of carbon dioxide, bicarbonate, carbonate, and hydroxide ions that are naturally present in water. – Dr Marc Sircus
Take the time to source a living high alkalising water. Remember the pH reading may be high but the ability to alkalise could be low. Poor diet, high sugar intake, stress, negative emotions and thinking, dehydration and an imbalanced over acid pH may lead to illness.
pH -water absorption and oxygenation work hand in hand, one missing link and the chain breaks.
Motivational Kinesiology has tools to test and potentially correct imbalances in the chain.





Leave A Comment
You must be logged in to post a comment.